Describe How Dilution Was Used to Prepare the Starch Solutions
Volume of diluted solution V2 50mL. X 25 ml of stock solution.
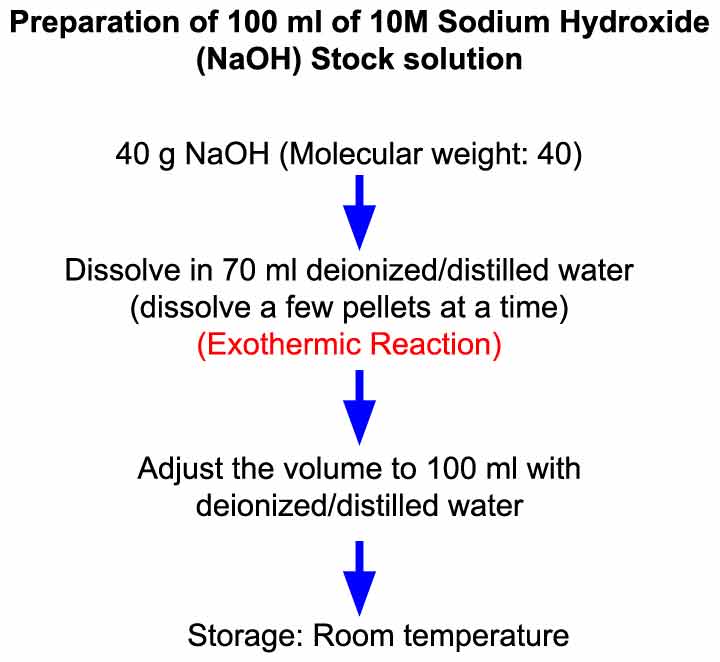
Preparation Of 10 M Sodium Hydroxide Naoh Solution Laboratory Notes
Volume of stock solution needed V1.

. C 2 Final concentration of new solution. The calculator uses the formula M 1 V 1 M 2 V 2 where 1 represents the concentrated conditions ie stock solution molarity and volume and 2 represents the diluted conditions ie desired volume and molarity. Reagent Preparation Practice ANSWERS Reminder.
There is now 1mL of the undiluted solution in 9 mL of the dilution liquid. Using the parts method if you merely add the two numbers of the parts ratio together and then divide that into 100 it will give you the final dilution percentage. Using the bench reagent commonly.
Swirl the flask and then top it up with more distilled water to the 10 mL mark. Table 1 starch solution dilutions dilution volume. The volume of the stock solution needed can be obtained by using the dilution formula as shown below.
When starch is heated in water decomposition occurs and beta-amylose is produced. V 2 Final volume of new solution. Plan how you will use the stock 2 starch solution to make the following five concentrations of starch solution.
If you take one part master solution and add four parts of distilled water you now have a 20 salt water solution 14 ratio. To prepare a solution of. Now 1 ml of mixture is taken from the 10 -1 dilution and is emptied into the second tube.
The pipette tip is discarded and a new pipette tip is attached to the pipette. A 500-mL sample of a 15 mv H2SO4 solution is added to water to give a final volume of 250 mL. To make your solution pour 25 ml of stock solution into a 50 ml volumetric flask.
V1 3 x 5012. A 100-mL sample of a 25 mv KOH solution is diluted with water so that the final volume is 1000 mL. Starch is a viable indicator in the titration process because it turns deep dark blue when iodine is present in a solution.
The solution dilution calculator tool calculates the volume of stock concentrate to add to achieve a specified volume and concentration. The dilution factor can be used to calculate the volumes and stock and diluent required in a particular instance. 2 Iodine solution Iodine is only sparingly soluble in water 03 g per litre.
Make 5 mL of a 025 M solution from a 1 M solution. Describe the dilution series used to make a 100-fold ie 1100 dilution a to prepare 250 ul of the diluted solution b to prepare 5 ml of the diluted solution. To prepare stock standard solution in lab you should first make sure that you understand the concentration unit principles and some mathematical rules which will help to find exact answer for reporting.
Your first step is to calculate the volume of stock solution that is required. V 1 Volume of stock solution needed to make the new solution. The dilution factor is obtained from the initial concentration of the stock solution and the fmal concentration of the diluted solution.
Pages 10 Ratings 100 1 1 out of 1 people found this document helpful. Shall be checked before use with another standard that has been prepared separately from different source. M dilution V dilution M stock V stock.
2 1 05 02 and 01 Use the waterproof pen to label five test tubes and five small beakers with the different starch concentrations. Perform the first dilution. To prepare the 10 mL of 2 M solution you must first transfer about 5 mL of distilled water into your 10 mL volumetric flask.
V1S1V2S2 So 5000 V1 01 100 V1 0002 mL Tr. How to do calculations for. It is usual to dissolve it in potassium iodide solution KI to make a 001 M solution by tenfold dilution of a 01 M solution to use as a starch test reagent.
The dilution factor is the total number of unit volumes in which your material will be. The solution therefore has been diluted by a factor of 10. View the full answer.
This preview shows page 3 -. Divide both side by 12. The volume of the stock solution needed is 125mL.
Fill the flask to the 1 L line. The dilution is thoroughly mixed by emptying and filling the pipette several times. Describe the dilution series used to make a 100-fold ie 1100 dilution a to prepare 250 ul of the diluted solution b to prepare 5 ml of the diluted solution.
10 M 50 ml 20 M x ml x 10 M 50 ml20 M. Infant formulas contain mixes of dextrins and maltose rather than starch because they are easier to. If a different molarity is required then multiply that number times the molar mass of NaCl.
Next slowly add your 4 mL of stock solution sulfuric acid. For example if you wanted a 05 M solution you would use 05 x 5844 gmol of NaCl in 1 L of solution or 2922 g of NaCl. The term used to describe a class of carbohydrates capable of transferring electrons to.
Molarity of diluted solution M2 3M. 1 Starch solution Add a weighed amount of starch 05 g or 10 g to a little heated water mix to a paste then dilute to 50 or 100 cm 3. Make sure that the starch is alpha and not beta amylase.
To prepare the solution. Add a small volume of distilled deionized water to dissolve the salt. 12 x V1 3 x 50.
Simple Dilution Dilution Factor Method based on ratios A simple dilution is one in which a unit volume of a liquid material of interest is combined with an appropriate volume of a solvent liquid to achieve the desired concentration. Course Title BIOL 1107. Starch is substrate for amylase enzyme and used 1 starch as substrate of enzyme assay Starch is insoluble in water If heat the solution or adding of starch in boiling water can be used to.
To make a fixed amount of a dilute solution from a stock solution you can use the formula. Water is added to 050 L of a 12 M NaOH solution to make 30 L of a diluted NaOH solution. This provides an initial dilution of 10 -1.
C 1 Concentration of stock solution. 952 grams in 1000mL So for 100mL we require 9521000100 952 g For 5mM we require 95210005 00476g NaCl. C 1 V 1 C 2 V 2 where.
Use freshly prepared starch solution for iodometric titrations. For example suppose you wanted to prepare 100mL of a solution of NaOH at 0lmolL-1. For example a starch-iodine titration can be used to determine.
How to Make Simple Solutions and Dilutions 1. M1V1 M2V2. Solution for Describe how you would prepare the required dilution series using the least amount of dilution water close.
Boil water and then add to the boiling water required starch. Start your trial now. Beta-amylose combines with iodine resulting in a dark blue color change.
School University Of Georgia. Draw 1 mL of undiluted solution from test tube US with a pipette and transfer it to the test tube labeled 110 containing 9 mL of the dilution liquid and mix thoroughly.
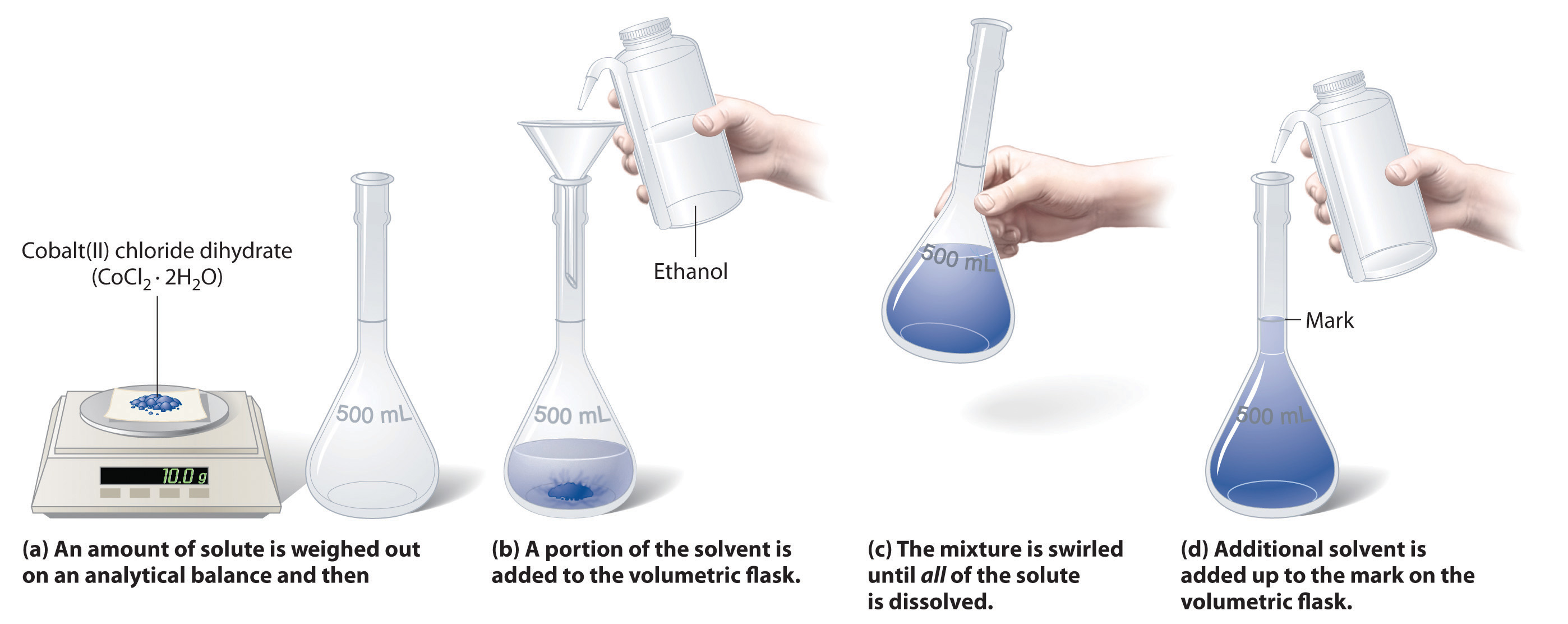
5 2 Solutions And Dilutions Chemistry Libretexts

Serial Dilution Methods Calaculations Youtube
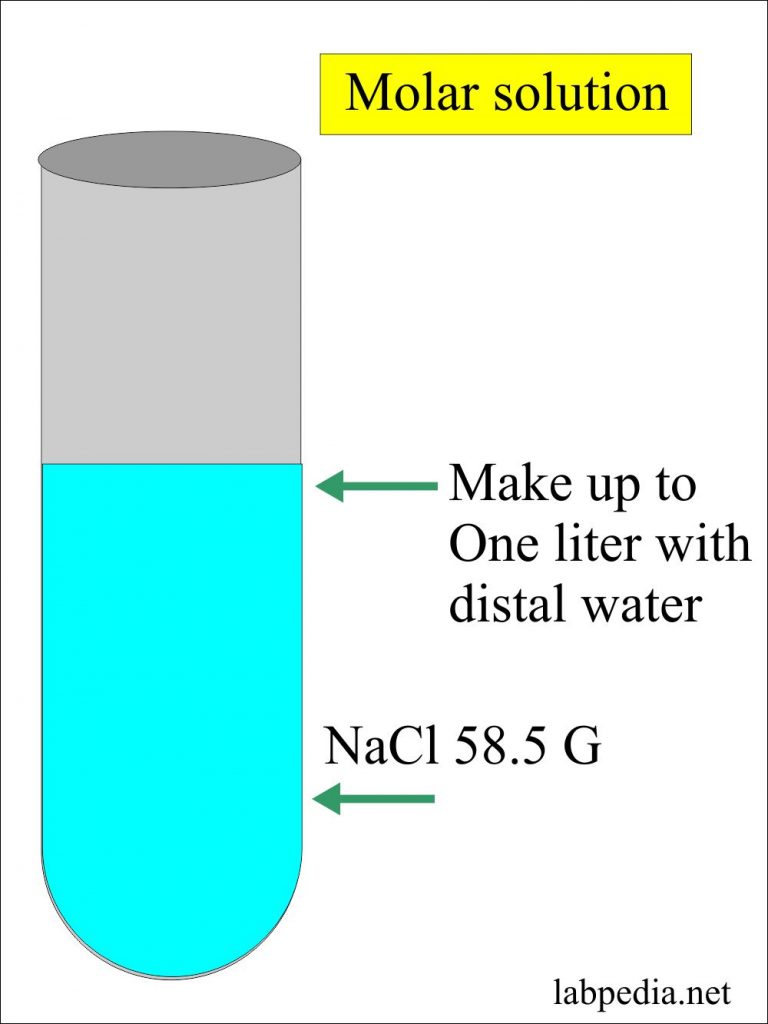
Solutions Part 2 Preparation Of Solutions Molar Normal And Dilution Labpedia Net
0 Response to "Describe How Dilution Was Used to Prepare the Starch Solutions"
Post a Comment